Anyone following NAMA and the industry’s work on calorie disclosure over the past six years could be easily confused with recent news coming out of the Food and Drug Administration (FDA) on nutrition labeling issues. The issues impacting food and beverage labeling include three important labeling rules either pending or finalized at FDA: disclosure of calories on products sold from vending machines, update of the iconic Nutrition Facts Panel (NFP) and menu labeling. NAMA has been actively involved in issues related to calorie disclosure and the NFP update, but not menu labeling. This article provides background on these three labeling issues and their impact on the convenience services industry.
Vending Calorie Disclosure
The Patient Protection and Affordable Care Act of 2010 (ACA), also known as Obamacare and made law in 2010, included vending machine labeling provisions establishing requirements for providing calorie declarations for food sold from certain vending machines. Following passage of the ACA, the FDA began the rulemaking process to help make calorie information for vending machine foods available to prospective purchasers in a direct, accessible, and consistent manner to enable them to making informed and healthful dietary choices. Specifically, the ACA and the rule require that vending machine operators who own or operator 20 or more vending machines much provide calorie declarations for those vending machine foods for which the Nutrition Facts label cannot be examined prior to purchase or for which visible nutrition information is not otherwise provided at the point of purchase.
In December 2014, the FDA issued a final rule settinga compliance date of December 1, 2016 for calorie disclosure for food sold from vending machines. The ultimate responsibility lies upon vending operators; however the industry has worked to together with the FDA and stakeholders to create reasonable flexibility in disclosing calories at the point of purchase to reduce the burden on both operators and food and beverage manufacturing companies. In 2016 FDA extended the compliance date for certain products sold in glass front vending machines to July 26, 2018, with the expressed intention to issue a proposed rule to addresses stakeholder concerns about technical challenges for implementation related specifically to font size of front of pack calorie disclosure; gum, mints and rolled candies calorie disclosure; and multi-serve packages disclosure. NAMA continues to advocate for a proposed rule to address these concerns before the July 26, 2018 deadline.
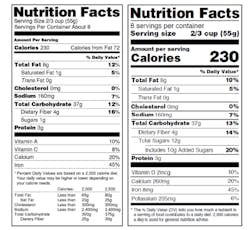
Nutrition Facts Label
On May 20, 2016, the FDA announced the new Nutrition Facts label for packaged foods to reflect new scientific information, including the link between diet and chronic diseases such as obesity and heart disease. The FDA stated that the new label will make it easier for consumers to make better informed food choices. The final rule became effective on July 26, 2016. The compliance date of this final rule was originally set for July 26, 2018 for manufacturers with $10 million or more in annual food sales and July 26, 2019 for manufacturers with less than $10 million in annual food sales.
In early May, the FDA announced that it was extending the compliance dates by approximately 1.5 years for providing updated nutrition information on the label of food, including dietary supplements; defining a single-serving container; requiring dual-column labeling for certain containers; updating, modifying, and establishing certain reference amounts customarily consumed; and amending the label serving size for breath mints.
Menu Labeling
On May 7th, the FDA announced that chain restaurants, grocery outlets, and convenience stores with more than 20 locations will be required to post caloric and nutritional information for food and drinks sold on-premise that are considered “standard menu items.” According to the FDA, a standard menu item is one that is routinely included on menus and menu boards.
Establishments covered by the rule must disclose the number of calories contained in standard items on menus and menu boards. For self-service foods and foods on display, calories must be listed in close proximity and clearly associated with the standard menu item. Furthermore, the FDA stated that businesses must also provide, upon request, the following written nutrition information for standard menu items: total calories; total fat; saturated fat; trans fat; cholesterol; sodium; total carbohydrates; sugars; fiber; and protein.